AGPase
Starch is important carbohydrate and the primary energy source for plants. Starch biosynthesis occurs by the participation of three mai enzymes: ADP - glucose pyrophosphate (AGPase), starch synthase and branching enzyme. The first enzyme, AGPase, in starch biosynthesis catalyzes the conversion of Glc-1-P and ATP to ADP-glucose and pyrophosphate (PPi). Starch synthase in next step uses ADP-glucose for synthesis of polyglucans. In higher plants AGPase act as a key regulatory allosteric enzyme in starch biosynthesis by catalyzing the rate limiting step. AGPase play main role in the modulation of photosynthetic efficiency in source tissues and in determining the level of starch storage in sink tissue, in turn influencing overall crop yield. Its regulation in almost all higher plants depends on the 3-phosphoglyceric acid to inorganic phosphate ratio (3PGA/Pi). Where 3GPA plays the role of main stimulator and Pi inhibits the enzyme activity.
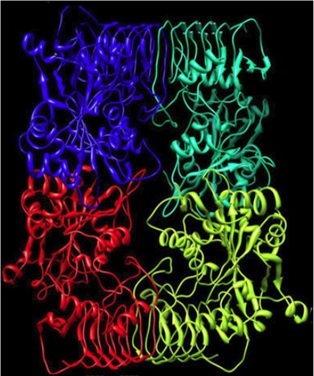
Plant AGPases are hetertertameric in structure with a pairs of samll (SS) and large(LS) subunits. Both SS and LS plays different role in enzyme functionality. SS has both ctalytic and regulatory functions as compared to LS which mainly responsible for regulation of allosteric properties for SS. Specific regions in both SS and LS plays a significant role in enzyme activity and subunit association. From 3-dimensional crystal structure of bacterial heterotetameric enzyme form A. tumefaciens and enzyme from potato tuber, it was deduced that enzyme functions as dimer of dimers.
Predicted Structure of AGPase Heterotetamer